Abstract
Adoptive cell therapy through T cells with Chimeric Antigen Receptor (CAR) including Tisagenlecleucel and Axicabtagene Ciloleucel introduced a breakthrough in cancer immunotherapy against CLL and have risen as game changers in the cancer care scenario but still remain several challenges including durable remission and lack of expansion after infusion. We previously discovered that CAR vector-mediated disruption of the epigenetic regulator TET2 resulted in the clonal expansion of CD8+ CAR T cells in a patient with Chronic Lymphocytic Leukemia (CLL) patient. Further, TET2 disrupted CAR T cells carried the immunophenotypic and epigenetic signatures of early memory T cells. Further, we demonstrated that small hairpin RNA (shRNA)-mediated TET2 knockdown in CAR T cells recapitulated the early memory phenotype. TET2 knocked-down CAR T cells also displayed augmented CAR antigen-triggered proliferation and cytokine polyfunctionality, which is in line with the augmented memory function. Since TET2 is a well-known oncogene the full ablation of this gene via CRISPR/Cas9 may result in the malignant transformation of the engineered T cells. We therefore sought to safely manipulate TET2 function via metabolic engineering. TET2 uses alpha-ketoglutarate (aKG) as a critical cofactor. Work from the Melnick lab (PMID: 21130701) had demonstrated that mutant IDH1 exhibits neomorphic activity and generates excess amounts of 2-hydroxyglutarate (2HG), a competitive inhibitor of TET2 activity. We hypothesized that the inhibition of TET2 function via the overexpression of this IDH1 neomorph in CAR T cells will recapitulate the genetic disruption observed in our patient.
We therefore generated a bicistronic expression system wherein anti-CD19 T cells co-expressed IDH1R132H(IDH1NM). As a control we overexpressed the wildtype IDH1 in CART19 cells. Overexpression of IDH1WT or IDH1NM in manufactured CAR19 T cells was confirmed via Western blotting. Further, IDH1NM overexpression in CAR19 T cells resulted in a significant accumulation of D-2-HG in IDH1NM but not IDH1WT overexpressing cells. Lastly, IDH1NM but not IDH1WToverexpressing CAR T cells exhibited significantly increased levels of cytosine methylation and reduced methyl-cytosine oxidation products the functional, indicating that our approach indeed interrupted TET2 function.
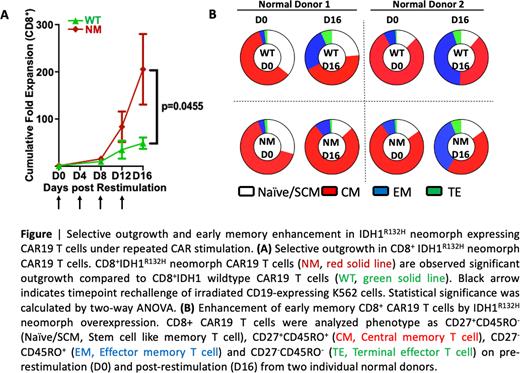
To examine the immunological impact of IDH1NM overexpression in CAR19 T cells, we tested IDH1NM CAR19 T cells and IDH1WT-CAR19 T cells with in vitro repeated CAR stimulation with CD19-expressing tumor cells as described in our previous study (Fraietta et. al. Nature 2018). We observed significant and selective outgrowth of CD8+ CAR19 T cells stimulated repeatedly at 4-day intervals with CD19+ tumor cells. The impact of IDH1NM on CD4+ CAR T cells was less pronounced. Also, we confirmed that this preferential outgrowth of CD8+ IDH1NM -CAR19 T cells is coupled with expansion of CD27+CD45RO- central memory subsets. As observed with shRNA-mediated TET2 knockdown we here observed that IFN-gamma levels were significantly reduced after the fourth stimulation in IDH1NM compared with IDH1WT cells. Importantly, IDH1NM overexpression had no impact on leukemia cell killing, indicating that memory cell function was augmented in CART19 cells with preservation of effector function. Lastly, IDH1NM overexpressing cells strongly depended on antigen stimulation for their proliferation. This study therefore suggests that the functional disruption of TET2 via the overexpression of the IDH1 neomorph results in augmented memory function of CAR T cells with preservation of effector function. Our discovery introduced an alternative approach for functional TET2 disruption for CAR T cell improvement.
Disclosures
Wang:Cambridge Epigenetix, Ltd.: Research Funding. Kohli:Life Edit, Inc.: Membership on an entity's Board of Directors or advisory committees; Cambridge Epigenetix, Ltd.: Membership on an entity's Board of Directors or advisory committees, Research Funding. Melenhorst:Novartis: Patents & Royalties: Biomarkers predictive of therapeutic responsiveness to chimeric antigens; Novartis: Patents & Royalties: Methods for improving the efficacy and expansion of immune cells; Chroma Medicine: Consultancy; Kite Pharma: Consultancy; Poseida Therapeutics: Consultancy; IASO Biotherapeutics: Consultancy, Research Funding; Novartis: Patents & Royalties: Methods of making chimeric antigen receptor - expressing cells; Novartis: Patents & Royalties: Methods for improving the efficacy and expansion of chimeric antigen receptor.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal